Transient Neurological Symptoms After
Isobaric Subarachnoid Anesthesia with 2% Lidocaine: The Impact of Needle Type

DOI:
10.1213/01.ane.0000281908.48784.91
ISSN: 0003-2999
Accession:
00000539-200711000-00052
Table of Contents:
≪ The Differential Effect of Cyclosporine on Hypnotic Response
and Pain Reaction in Mice.
≫ Recovery
Profiles of General Anesthesia and Spinal Anesthesia for Chemotherapeutic
Perfusion with Circulatory Block (Stop-Flow Perfusion).
Search Results:
≫ Pioneers
in Epidural Needle Design.
Links
·
Abstract
·
METHODS
·
RESULTS
o
APPENDIX
Graphics
·
Figure
1
·
Table
1
·
Table
2
·
Table
3
BACKGROUND: The reported incidence of
transient neurological symptoms (TNS) after subarachnoid
lidocaine administration is as high as 40%. We
designed this clinical trial to determine the incidence of TNS with two
different pencil-point spinal needles: one-orifice (Atraucan)
and two-orifice (Eldor) spinal needles.
METHODS: Ninety-nine ASA physical
status I or II patients undergoing surgical procedures of the urinary bladder
or prostate were prospectively allocated to receive spinal anesthesia with 40
mg, 2% isobaric lidocaine plus fentanyl
injected through either a 26-gauge Atraucan (n
= 52) or a 26-gauge Eldor (n = 47) spinal
needle. During the first three postoperative days, patients were observed for
postoperative complications, including TNS. The primary end-point for this
trial was the percentage of TNS in both double- and single-orifice spinal
needle procedures.
RESULTS: The incidence of TNS was
higher when spinal anesthesia was done through the Atraucan
needle (28.8% vs 8.5%, P = 0.006). Fifty
percent of the patients in the double-orifice group versus 100% of the
single-orifice group developed TNS after surgery in the lithotomy
position (P = 0.014). The relative risk for developing TNS with the Eldor needle was 0.29 (95% CI: 0.07–0.75) compared with the
Atraucan needle.
CONCLUSIONS: The use of a
double-orifice spinal needle was associated with a lower incidence of TNS,
which may have been due to the needle design.
Transient neurological symptoms (TNS) are characterized
by postoperative pain or dysesthesia in the buttocks
or lower extremities. The cause of TNS has been investigated in several studies
in association with lidocaine concentration (1), osmolarity
(2),
dextrose concentration (2), lithotomy
position (3),
ambulatory surgery (3),
and early ambulation (4).
The only factors found to be associated with increased risk of TNS were lidocaine spinal anesthesia, the lithotomy
position, and ambulatory surgery (4). Pooling and maldistribution of local anesthetic encountered with the
use of pencil-point spinal needles (5) or spinal microcatheters (6) were suggested to have
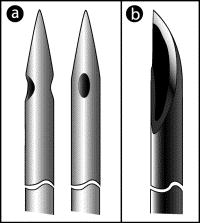
a causative effect for neurological injury, but not TNS. The Eldor spinal needle (Fig. 1)
has two rounded opposing lateral orifices at its pencil-point tip (7) that might affect the
dispersion of local anesthetic. However, a single study comparing the block
characteristics achieved by this needle versus a single-orifice Sprotte needle found no differences in the quality of the
block (8).
In this randomized single blinded comparison study using 2% lidocaine
in patients undergoing surgical procedure of the urinary bladder or prostate,
we evaluated the newly shaped Eldor spinal needle
specifically with regard to the incidence of TNS.
|
Figure 1. Spinal needle types. (a) Double-orifice: Eldor needle. (b) Single-orifice: Atraucan.
|
After obtaining approval of the IRB and
written patient informed consent, 99 ASA physical status I and II patients
scheduled for suprapubic prostatectomy or
transurethral resection of prostate, under spinal anesthesia, were randomized
blindly to receive spinal anesthesia with 40 mg, 2% plain preservative-free lidocaine (Rafa Laboratory,
Jerusalem, Israel) plus 15 µg fentanyl (Janssen Cilag, Kibbutz Shefaim, Israel),
injected through either a 26-gauge pencil-point, Eldor
needle with two lateral opposing orifices at its tip (TSK Laboratory, Baldoyle Ind, East Dublin,
Ireland) or a 26-gauge, single-orifice Atraucan
cutting-point double-bevel needle (B. Braun, Melsungen
AG, Germany). Randomization was performed using a cluster design, in which
patients belonging to a specific session and undergoing a urology procedure of
prostate or urinary bladder were given spinal anesthesia with the same type of
needle. Patients were randomly allocated to have their spinal anesthesia
performed by one of the two types of needles. Needles were kept in sealed
envelopes marked with the session number, using random number tables.
Excluded from the study were patients
with a history of chronic pain, presence of neurological disease, diabetes
mellitus, smoking of more than 10 cigarettes per day for more than 3 yr,
chronic use of analgesic medications, and body mass index >30.
Premedication consisted of oral diazepam, 7.5 mg, 1
h before surgery. Intraoperatively, monitors were
applied as per ASA guidelines. Before spinal anesthesia was performed, 10 mL/kg of lactated Ringer's solution was administered IV
over 20 min. With the patient in the sitting or left lateral decubitus position, the patient's skin was prepped with a
10% solution of povidone iodine in isopropyl alcohol
(Fisher Pharmaceutical Labs, Tel Aviv,
Arterial blood pressure was recorded at
1 min intervals for the first 20 min and at 5 min intervals thereafter, until
complete (motor and sensory) recovery from spinal anesthesia. Hypotension was
defined as a decrease in systolic blood pressure of >
The primary outcome for this trial was
the percentage of TNS in both double- and single-orifice spinal needle
procedures. Initially, the sample size was based on one planned analysis at the
end of the study. A sample size of 150 patients in each group was calculated to
achieve 80% power and a two-sided [alpha] of 0.05, assuming a 12.5% and
25% incidence of TNS in the double- and single-orifice groups, respectively.
After 99 patients were enrolled in the study, a safety concern was raised by
the physicians who were involved in data collection due to the clinical
impression of frequent TNS. Although the overall incidence of TNS was not
increased, the possibility of a large group disparity raised ethical concerns
that led to the decision of data unblinding. Before unblinding the data, the statistician and the IRB were
informed regarding our clinical suspicion of frequent TNS. The course of action
decided at that stage was to terminate the study in case of verification of a
significantly frequent incidence of TNS in the interim analysis. Interim
analysis at that point revealed a 19% overall incidence of TNS with 8.5% and
28.8% in the double- and single-orifice groups, respectively. Because of
ethical concerns, a decision was then made to terminate the study.
The difference in TNS rates in the
collected data was then analyzed by group sequential (interim) analysis
methods, and results were found to be consistent with a statistically
significant difference of >17.8% between the two groups. The interim
analysis was performed by a statistician who decided on the criteria by which
the study would be discontinued. This boundary was calculated based on the
previously specified power, significance level, and effect sizes. The boundary
shape (determined after data unblinding) was set
according to Pocock (9). Such an approach
requires a predetermined number of interim analyses. Because such a judgment
had not been determined at the study design phase, the number of analyses was ad
hoc set to four. These calculations led to a maximal sample size of 200
patients in each group, with four interim analyses, after enrollment of 50
patients per group. The number of patients in each group at time of study
termination matched the number of patients needed for first data analysis (
The boundaries for achieving a
two-sided statistical significance of 0.05 were a difference in TNS rate of
17.8%, 12.6%, 10.3%, and 8.9% in each interim analysis, respectively. For
further details, see Jennison and Turnbull (10). Adjustments to the P
value and the confidence interval, comparing TNS rates, were done using
analysis time ordering.
Univariate comparisons of the patients' characteristics were
performed using unpaired t-test, [chi]2,
or Fisher's exact test. The Fisher's exact test was used when the expected values
in a cell were less than five. Stratified analysis and Mantel-Haenszel relative risk and 95% confidence intervals were
used to examine potential confounders. For variables significantly different
between groups in the univariate analysis, odds
ratios and 95% confidence intervals were obtained from multiple logistic
regression analysis to estimate their independent contribution to TNS. The
module used for statistical analysis was Seq Trial 2
(2002) in 5-Plus 6, 2 ©, 2003, Insightful Corp.
During the 6-mo study period, 152
patients underwent urology procedures under spinal anesthesia. Of them, 99
(65.1%) fulfilled the inclusion criteria and agreed to participate in the
study. Excluded from the study were patients with diabetes mellitus (14.5%),
obesity (8.6%), technical difficulties in performing spinal anesthesia (4.6%),
refusal to participate (3.3%), neurological problems (2%), and other reasons
(2%). There were no differences in the patients' demographics, surgery
characteristics and quality, and duration of spinal anesthesia (Table 1).
There were 47 procedures performed with the Eldor
needle and
|
Table 1. Demographic and Clinical Characteristics of Patients
According to the Type of Needle Used for Spinal Anesthesia |
|
Table 2. Symptoms Reported Within 72 h of Surgery by Type of
Needle Used for Spinal Anesthesia |
|
Table 3. Odds Ratio (95% Confidence Interval) of the
Association Between the Type of Needle, Type of Surgery, and Positioning
During Spinal Anesthesia, and Transient Neurological Symptoms |
Our study suggests that TNS is more
common after spinal anesthesia with 2% isobaric lidocaine,
plus fentanyl, when a single-orifice cutting point (Atraucan) needle is used in patients undergoing urology
procedures. Our report on the incidence of TNS is similar to the previously
reported incidence with lidocaine (14.2%) and prilocaine (4%) for the same type of surgery with spinal
anesthesia performed through 25-gauge to 29-gauge Quincke
needles (11).
Other studies report an incidence of TNS ranging from 4% to 37% (12–17). Although most
local anesthetics are associated with TNS (12,18–23),
it occurs most frequently with lidocaine. The type
and duration of surgery and patient position during surgery may also add to the
variability of the factors implicated with the TNS (3,12,14,17). All these
variables were assessed in our study and were not found to be confounding for
the association between the needle type and incidence of TNS. The precise
etiology of TNS has not been elucidated, with previous theories having
postulated local anesthetic toxicity (17,24–26),
needle trauma or neuronal ischemia as a result of sciatic stretching (27).
Pooling and maldistribution
of local anesthetic, encountered with the use of pencil-point needles, was also
suggested to cause transient neurologic deficit, but
not TNS (5).
This was suggested to occur after slow injection rates of 5% hyperbaric lidocaine with sacrally directed Whitacre needles, or when using spinal microbore
catheters (6).
Freedman et al. (3),
in a large epidemiologic study of needle types (Quinke
versus Whitacre), found no difference in the rate of
TNS, although both were single-orifice needles with different distributions of
local anesthetic. We undertook our study with the hypothesis that the type of
the needle may affect the incidence of TNS and that, specifically, the
two-orifice needle may produce a more uniform distribution of the local
anesthetic. However, when comparing the two-orifice, 26-gauge Eldor spinal needle, with the 27-gauge Pencan
(Sprotte) needle (8), there were no
differences in the quality of the block consistent with our results. Although
neural injury is clearly linked to anesthetic maldistribution,
there was no evidence supporting the link with TNS in previous studies or the
current study. For example, in our study, the maximum sensory level was similar
between the groups, implying that the maldistribution
of local anesthetics does not seem to be the cause of the different incidence
of TNS between the groups.
A limitation of our study is the lack
of homogeneity of the groups in regard to type of surgery and patient's
position during the spinal administration. The difference in operative
procedures stems from the nonhomogeneity of the
patients in the cluster. However, despite the statistical significance of these
differences when reported as univariate variables,
the multivariable analysis did not identify these two variables (type of
surgery and patient's position) as confounding factors when comparing the type
of needle and the incidence of TNS. Also, methodology bias cannot be excluded
by early termination of our study and the performance of interim analysis of
the results.
The interim analysis was not planned at
the study design, but was decided upon due to concerns regarding a potentially
high disparity in the incidence of TNS. This necessitated the interim analysis.
Statistically, it has not been determined how to address the problem of
unplanned interim analysis (10). After unblinding the data, we constructed a group sequential
study (interim analysis design) to match the enrolled patients' status with the
previously specific study parameters. This approach allowed us to conduct an ad
hoc interim analysis as if it were initially planned. We believed that this
approach was suboptimal, but it yielded lesser bias than disregarding the fact
of early study termination. As for the P value, the boundaries were 17%
when comparing the TNS incidence for a significance level of 0.05. The adjusted
P value comparing TNS incidence was P value = 0.006 with adjusted
95% CI of the difference in TNS rate of 5.6%–35%. Finally, our study was not
double-blind. However, although the anesthesiologist performing spinal
anesthesia was not blinded, both the investigator and the patient were unaware
of the needle type used for spinal anesthesia. Therefore, it is unlikely that
an investigator bias affected the results of the study. In our study, we used
two types of needles: the pencil-point needle has been shown to be associated
with maldistribution of photalocyanine
blue dye in a spinal model, whereas the Quincke
needle was not (5).
The results of our study show that the
use of a single-orifice Atraucan spinal needle for
the administration of 40 mg of 2% spinal lidocaine in
urology surgeries was associated more frequently with TNS when compared with
the use of the two-orifice Eldor needle. The reduced
incidence of TNS with the use of two-orifice spinal needle may be attributable
to the needle design.
APPENDIX [Context Link]![]()
|
Table. No
caption available. |
1. Pollock JE, Liu SS,
Neal JM, Stephenson CA. Dilution of spinal lidocaine does not alter the incidence of transient neurologic symptoms. Anesthesiology 1999;90:445–50 Ovid Full Text Bibliographic Links
[Context Link]
2. Hampl
KF, Schneider MC, Ummenhofer W, Drewe
J. Transient neurologic symptoms after spinal
anesthesia. Anesth Analg
1995;81:1148–53 Ovid Full Text Bibliographic Links
[Context Link]
3. Freedman JM, Li DK, Drasner K, Jaskela MC, Larsen B, Wi S. Transient neurologic
symptoms after spinal anesthesia: an epidemiologic study of 1,863 patients.
Anesthesiology 1998;89:633–41 [Context Link]
4. Lindh
A,
5. Beardsley D, Holman
S, Gantt R, Robinson RA, Lindsey J, Bazaral M,
Stewart SF, Stevens RA. Transient neurologic deficit
after spinal anesthesia: local anesthetic maldistribution
with pencil point needles? Anesth Analg
1995;81:314–20 Ovid Full Text Bibliographic Links
[Context Link]
6. Rigler
ML, Drasner K. Distribution of catheter-injected
local anesthetic in a model of the subarachnoid
space. Anesthesiology 1991;75:684–92 [Context Link]
7. Eldor
J. Double-hole pencil-point spinal needle. Reg Anesth 1996;21:74–5 [Context Link]
8. Puolakka
R, Haasio J, Rosenberg PH, Tuominen
M. Comparison of double-hole and single-hole pencil-point needles for spinal
anesthesia with hyperbaric bupivacaine. Reg Anesth Pain Med 1998;23:271–7
Bibliographic Links
[Context Link]
9. Pocock
SJ. Group sequential methods in the design and analysis of
clinical trials. Biometrika 1977;64:191–9 Bibliographic Links
[Context Link]
10. Jennison
C, Turnbull BW. Group sequential methods with application to
clinical trials.
11. Ostgaard G, Hallaraker O, Ulveseth OK, Flaatten H. A randomised study of lidocaine
and prilocaine for spinal anaesthesia.
Acta Anaesthesiol Scand
2000;44:436–40 Buy Now Bibliographic Links
[Context Link]
12. Pollock JE, Neal
JM,
13. Hampl KF, Schneider MC, Pargger
H, Gut J, Drewe J, Drasner
K. A similar incidence of transient neurologic
symptoms after spinal anesthesia with 2% and 5% lidocaine.
Anesth Analg 1996;83:1051–4
Ovid Full Text Bibliographic Links
[Context Link]
14. Hampl KF, Schneider MC, Thorin D,
Ummenhofer W, Drewe
J. Hyperosmolarity does not contribute to transient radicular irritation after spinal anesthesia with
hyperbaric 5% lidocaine. Reg
Anesth 1995;20:363–8 Bibliographic Links
[Context Link]
15. Liguori GA, Zayas VM, Chisholm
MF. Transient neurologic symptoms
after spinal anesthesia with mepivacaine and lidocaine. Anesthesiology 1998;88:619–23 Ovid Full Text Bibliographic Links
[Context Link]
16. Martinez-Bourio R, Arzuaga M, Quintana JM,
Aguilera L, Aguirre J, Saez-Eguilaz JL, Arizaga A. Incidence of transient neurologic
symptoms after hyperbaric subarachnoid anesthesia
with 5% lidocaine and 5% prilocaine.
Anesthesiology 1998;88:624–8 Ovid Full Text Bibliographic Links
[Context Link]
17. de Jong RH. Last round for a “heavyweight”?
Anesth Analg 1994;78:3–4 Ovid Full Text [Context Link]
18. Drasner K, Rigler ML, Sessler DI, Stoller ML. Cauda equina syndrome following
intended epidural anesthesia. Anesthesiology 1992;77:582–5
[Context Link]
19. Pollock JE.
Transient neurologic symptoms: etiology, risk
factors, and management. Reg Anesth
Pain Med 2002;27:581–6 Bibliographic Links
[Context Link]
20. Salazar F, Bogdanovich A, Adalia R, Chabas E, Gomar C. Transient neurologic symptoms after spinal anaesthesia
using isobaric 2% mepivacaine and isobaric 2% lidocaine. Acta Anaesthesiol Scand 2001;45:240–5 Buy Now Bibliographic Links
[Context Link]
21. Ganapathy S, Sandhu HB, Stockall CA, Hurley D. Transient neurologic
symptom (TNS) following intrathecal ropivacaine. Anesthesiology 2000;93:1537–9 Ovid Full Text Bibliographic Links
[Context Link]
22. Lewis WR, Perrino AC Jr. Transient neurological symptoms after subarachnoid meperidine. Anesth Analg 2002;94:213–4 Ovid Full Text Bibliographic Links
[Context Link]
23. Drasner K. Chloroprocaine spinal
anesthesia: back to the future? Anesth Analg 2005;100:549–52 Ovid Full Text [Context Link]
24. Schneider M, Ettlin T, Kaufmann M, Schumacher P, Urwyler
A, Hampl K, von Hochstetter
A. Transient neurologic toxicity after hyperbaric subarachnoid anesthesia with 5% lidocaine.
Anesth Analg 1993;76:1154–7
Ovid Full Text Bibliographic Links
[Context Link]
25. Drasner K. Lidocaine spinal
anesthesia: a vanishing therapeutic index? Anesthesiology 1997;87:469–72 [Context Link]
26. Hodgson PS, Neal
JM, Pollock JE, Liu SS. The neurotoxicity
of drugs given intrathecally (spinal). Anesth Analg 1999;88:797–809 Ovid Full Text Bibliographic Links
[Context Link]
27. Salmela L, Aromaa U, Cozanitis DA. Leg and back pain after spinal anaesthesia involving hyperbaric 5% lignocaine.
Anaesthesia 1996;51:391–3 Buy Now [Context Link]